Pfizer, Moderna and Johnson & Johnson lead vaccination efforts across the US
May 5, 2021
Throughout these crazy times, what everyone wants is a way out. With hopes for the pandemic to come to an end, vaccines might be the answer.
In the United States, there are currently three FDA-approved COVID-19 vaccines: Pfizer-BioNTech, Moderna and Johnson & Johnson. However, there are additional vaccines that have been approved in other countries.
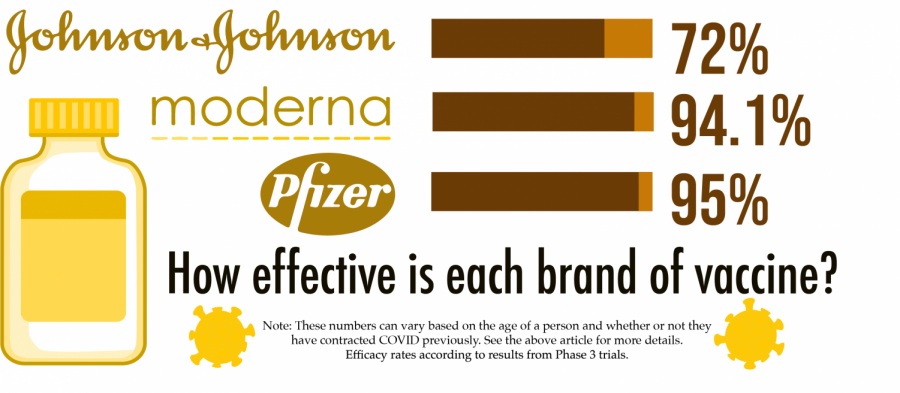
The first vaccine to be approved by the FDA was the Pfizer-BioNTech vaccine, which was authorized on Dec. 11, 2020. This vaccine is approved for patients over the age of 16 and has been proven to be equally effective across various demographics. It is an mRNA based vaccine. There are two doses for this vaccine, with each shot given with a 21-day period in between. So far, this vaccine has a 95% efficiency rate and is very popular. The most common short-term side effects of the Pfizer-BioNTech vaccine include: are, chills, headache, pain, tiredness, and pain at the injection site.
This vaccine however, is particularly hard to store because it has to be stored at minus 94 degrees to work effectively. This is the reason why it is particularly hard to transport and maintain compared to the other vaccines. Due to those hurdles, it is used in larger urban areas and megasites rather than smaller pharmacies.
The second vaccine to be approved was Moderna’s, authorized for emergency use about a week after Pfizer, on Dec. 18, 2020. This vaccine was found to be 94.1% effective in preventing symptoms within people who have not been previously exposed to COVID-19 and 86% effective in patients 65 and older. It is easier to store since it lasts in standard freezer temperature for 30 days. The dosage consists of two shots, given 28 days apart.
As another mRNA vaccine, this vaccine is similar to Pfizer. It uses the same technology and has similar effectiveness in preventing symptomatic disease. Side effects are very similar to Pfizer and consist of the same flu-like symptoms.
The Johnson & Johnson vaccine, authorized on Feb. 27, 2021, is the third and most recent vaccine to be approved in the U.S. thus far.
Unlike the Moderna and Pfizer vaccines, it is not mRNA. Although this vaccine is new and is using a different approach than the others, it has proven to show great results with a 72% efficiency rate and 86% efficiency rate against severe disease. Though it is approved for adults 18 years and older, Johnson & Johnson announced that they will start testing on children soon. This vaccine is easier to store and administer because it is kept at a refrigerator temperature and only requires one shot. Because it is easier to store and only requires one shot, it is favored by small communities as well as the homeless.
Johnson & Johnson is a carrier vaccine, which means it uses a different method than aforementioned mRNA vaccines. This vaccine is used to instruct human cells to make the coronavirus spike protein. A harmless adenovirus is injected as a shell which then carries a genetic code on the spike proteins to the cells. These codes and shells will train your cells to produce antibodies to protect the body from the virus.
On Tuesday, April 13 the Johnson and Johnson vaccine paused distribution of all injections. The source of the pause occurred when six patients developed a rare blood-clotting disease within weeks of their vaccination. Distribution of the Johnson and Johnson vaccine has since been resumed.
The common symptoms vary slightly compared to the Moderna and Pfizer vaccines and include fatigue, fever, headache, injection site pain, or myalgia.
Senior Lynne Walenjus of Wall received the Pfizer vaccine this past year. At the time, the Pfizer BioNTech vaccine was the only one she was eligible for.
Walenjus works at a dental office in Manasquan and when the vaccine became available to healthcare workers, she decided to get it and face the possible side effects.
“I was nauseous the night of the first dose,” Walenjus said. “After the second dose, I had a very high fever and chills, but they only lasted one day.”
Health and science teacher Leah Morgan also received the Pfizer-BioNTech vaccine. Morgan got the vaccine due to having pre-existing medical conditions that made her more vulnerable to COVID-19.
“I also know that the country will not even approach any form of normalcy if the majority of the population does not get vaccinated,” Morgan said.
With the first dose, she explained that she had a very sore arm for two days and after the second dose, she received flu-like symptoms and was sick for 36 hours.
“Although I know that the vaccine is not 100% effective and it doesn’t prevent all illness it goes a long way to mitigating the spread of COVID. We still need more research to determine if vaccinated people are 100% not carriers or spreaders of the disease and once almost everyone is vaccinated the country can return to close to normal,” Morgan said.